Top Notch Info About How To Tell If An Atom Is Polar
If the molecule has more than one element.
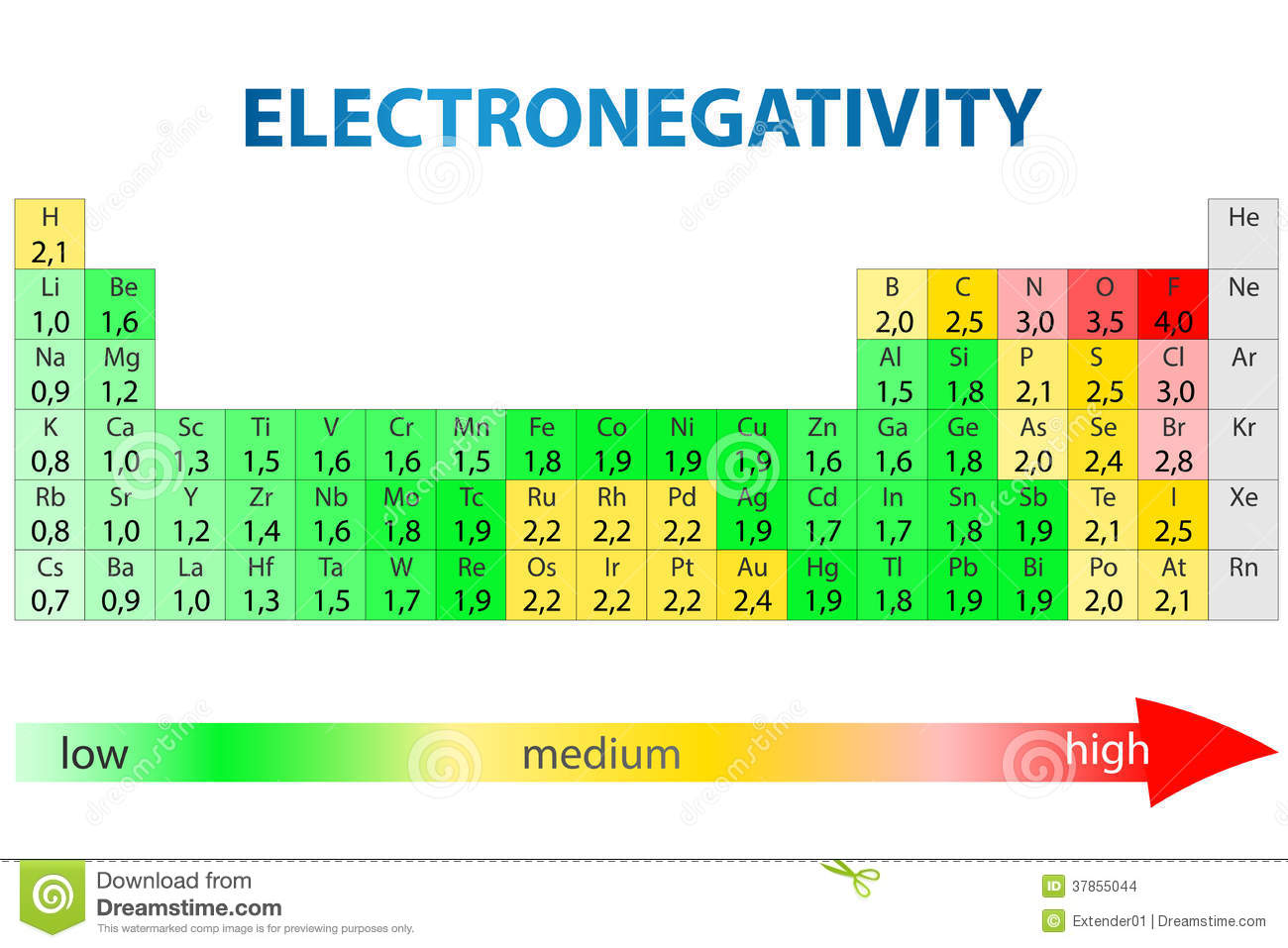
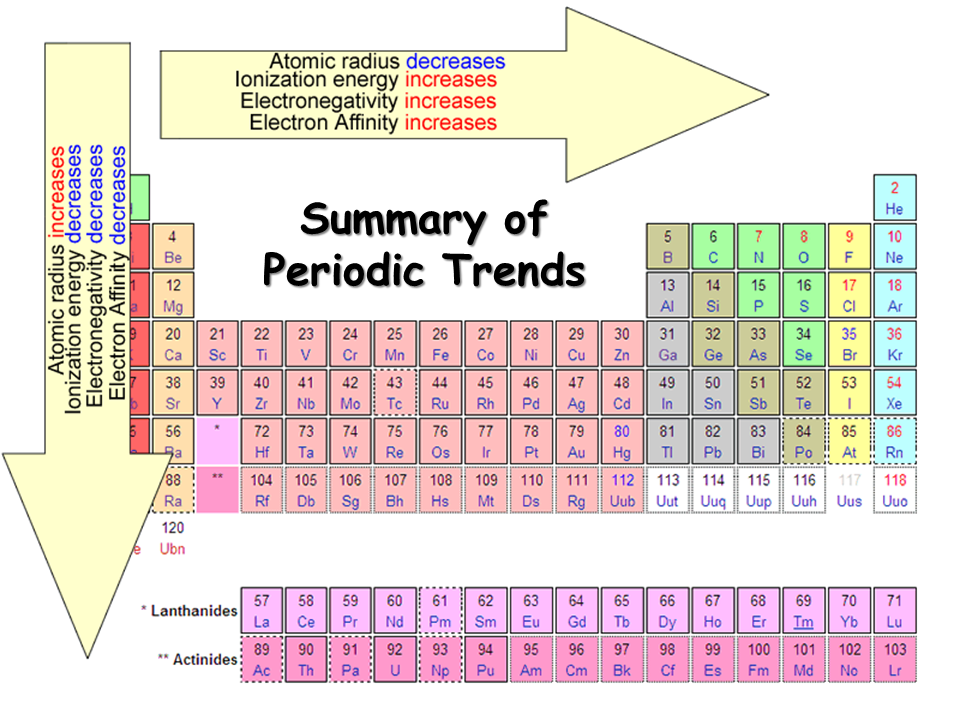
How to tell if an atom is polar. As aforesaid, bent molecules are. It is a measure of an atom's ability to attract and hold onto electrons within a chemical bond. A polar covalent bond is a.
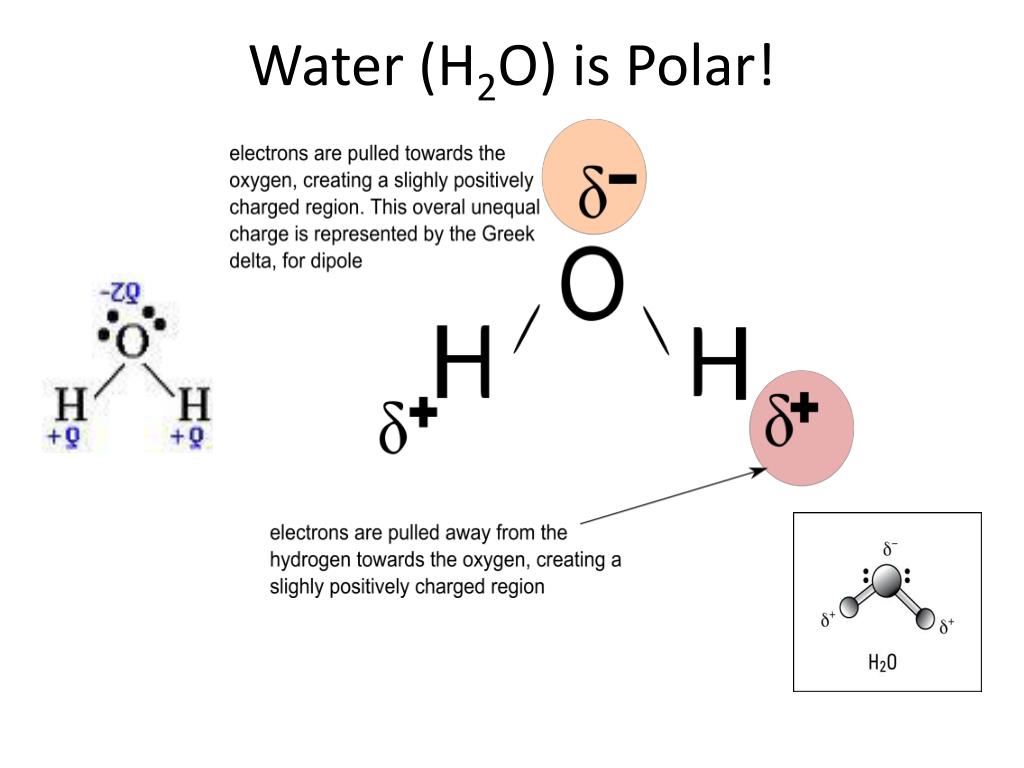
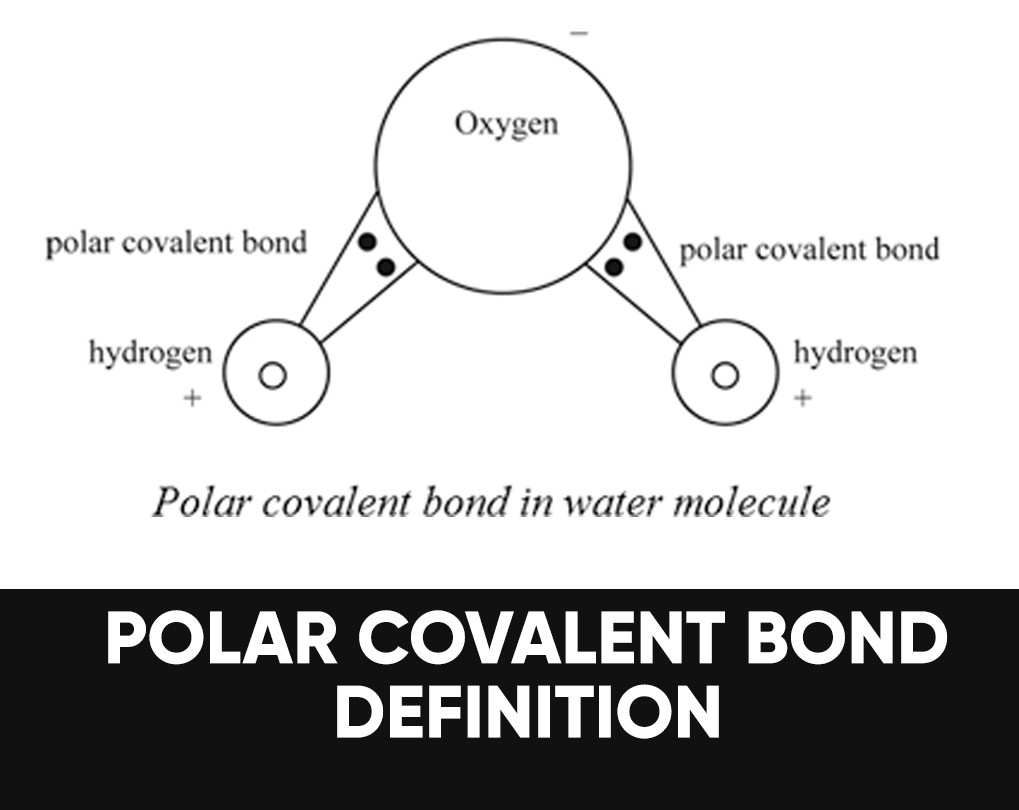
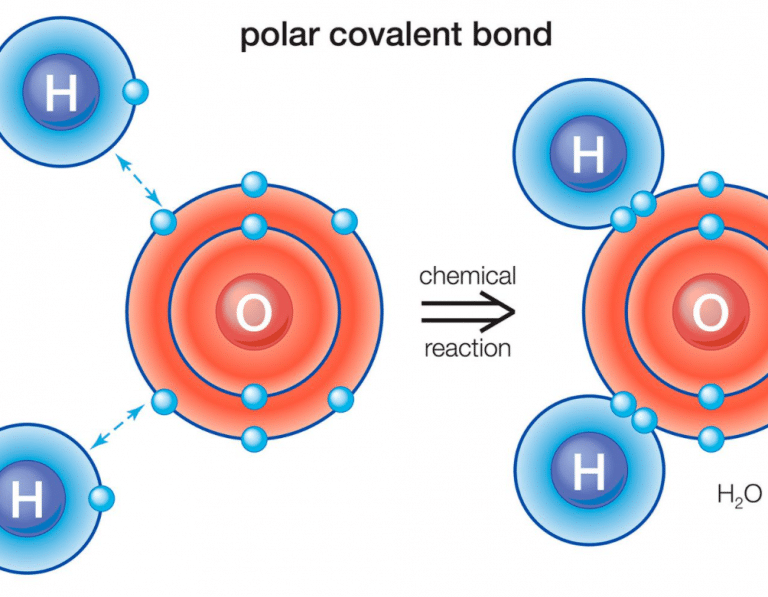
Further aspects of covalent bonding. The individual dipoles point from the h atoms toward the o atom. In a polar molecule, electron density is unevenly distributed throughout the.
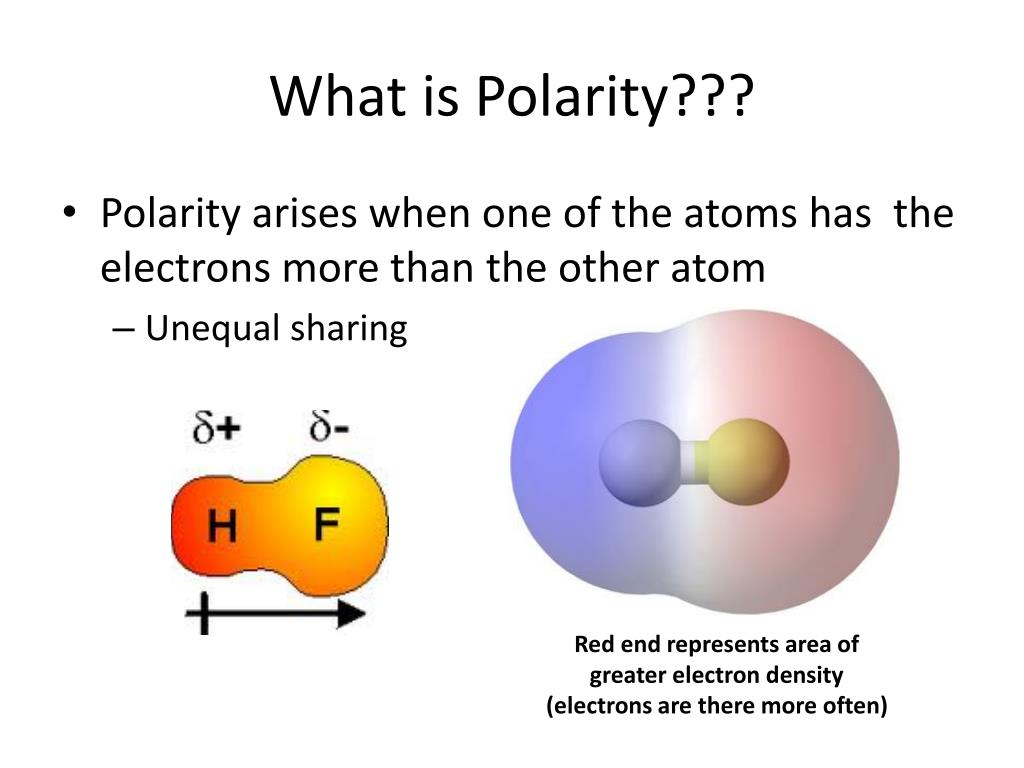
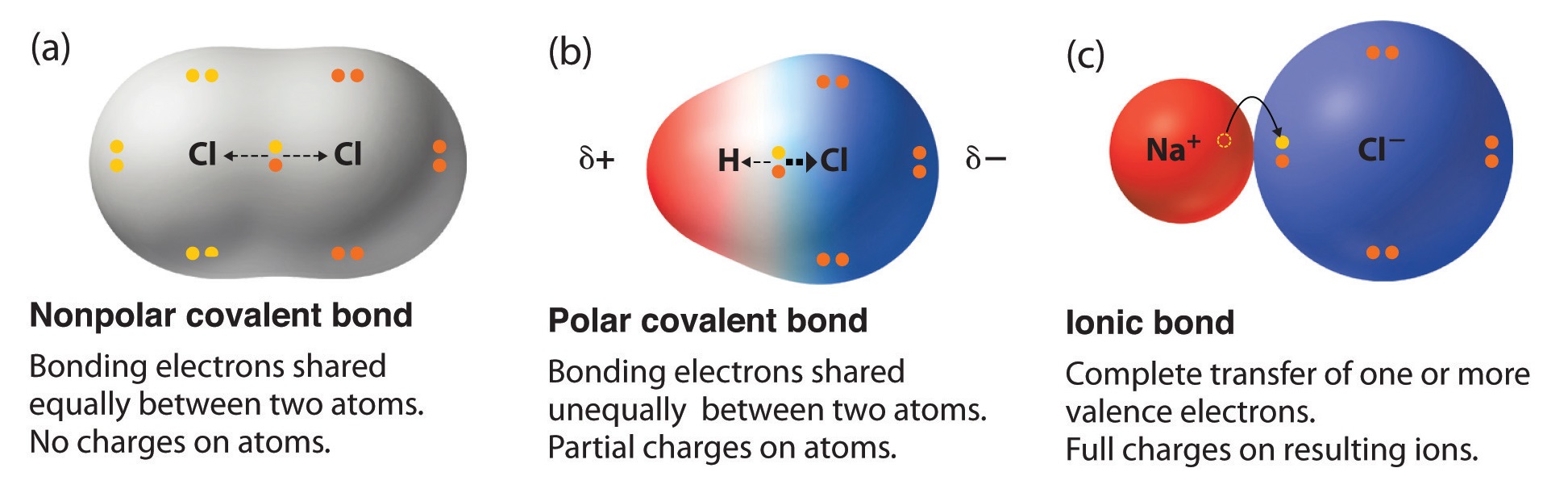
If one end of the molecule has a positive charge while the other end has a negative charge, the molecule is polar. If different kinds of atoms are attached to the central atom, the molecule is polar. Some bonds between different elements are only.
If a charge is evenly distributed around a central. This video provides a fast way for you to determine if a molecule is polar or nonpolar. This video discusses how to tell if a molecule / compound is polar or nonpolar.
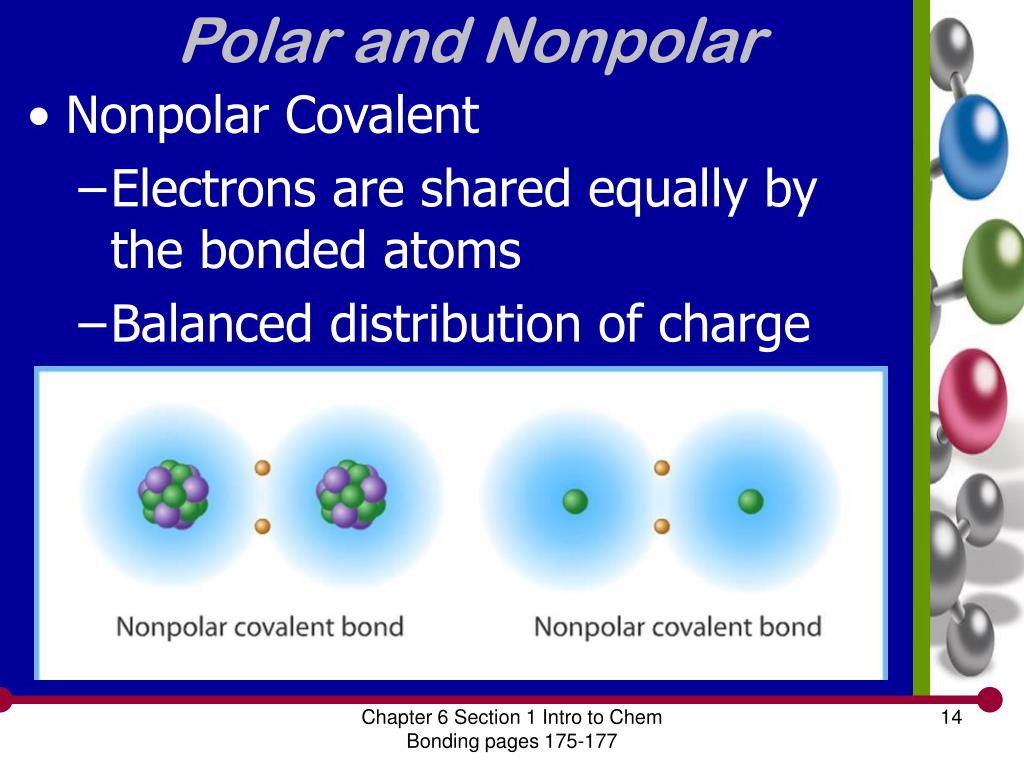
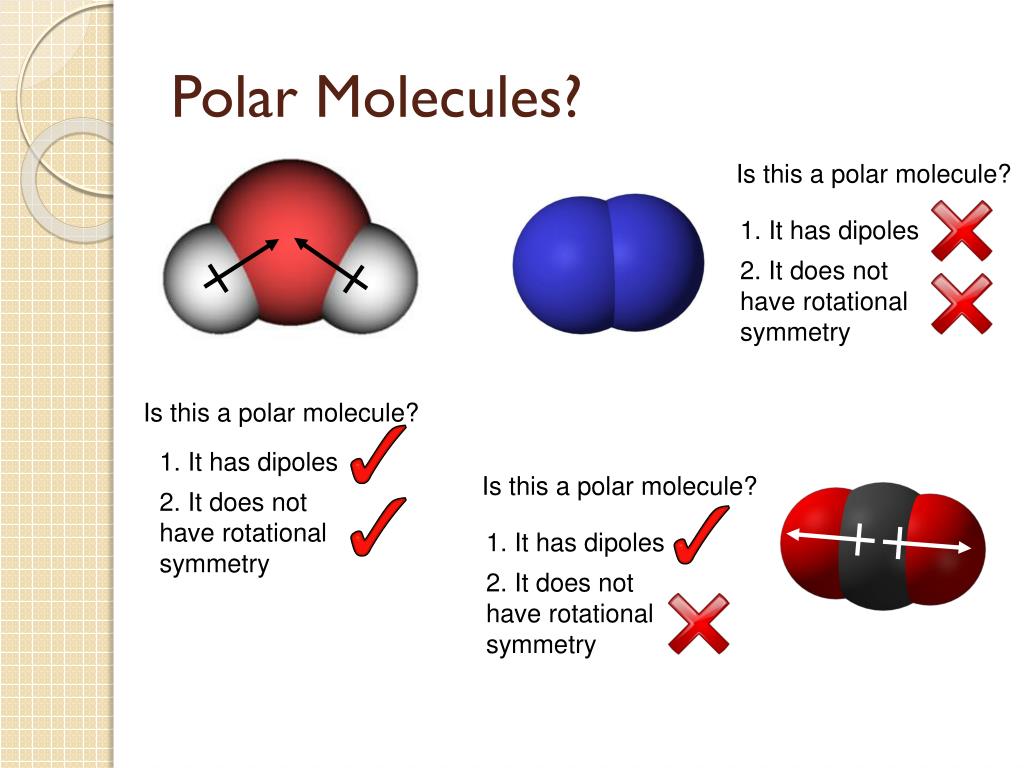
The polarity of a molecule tells whether the electron cloud is equally distributed across the atoms within the molecule, or whether an electronegative atom is affecting the electron. Like bonds, molecules can also be polar. Because of the shape, the dipoles do not cancel each other out and the water molecule is polar.
Polar atoms occur when different atoms are bonded within the molecule, such as carbon dioxide (co2) and water (h2o), where the pull of certain atoms cause the electron distribution to become unequal. When evaluating the polarity of a molecule, the electronegativity values. Are all bent molecules polar?
It provides examples so you can quickly distinguish nonpolar molecul. If a molecule has more than one polar bond, the molecule will be polar or nonpolar, depending on how the bonds are arranged. Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom and/or bonded to atoms that have different electronegativities.
If the electronegativity between the two atoms is between 0.5 and 2.0, they will. However, if one of the peripheral h atoms is replaced with another atom that has a different electronegativity, the molecule becomes polar. Chemprime (moore et al.) 7:
By sean lancaster. Any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. The molecule as a whole will also be polar.






/PolarConvalentBond-58a715be3df78c345b77b57d.jpg)